Vorstellung der Kundenchallenge
Erstes kennenlernen und Gewinnung Verständnis der Problemstellung. Kurzvorstellung der TopEase®-Plattform.
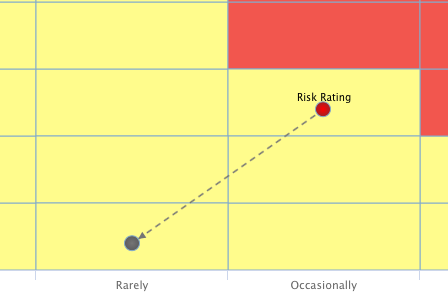
Analyse des Problemstellung
Analyse der Ist-Situation anhand der individuellen Finanzierungs- und Produktsituation. Leaner Ansatz, Fragestellungen z.B. bzgl. Produktroadmap (Planung DiGa / DiPa) und größter Handlungsbedarfe im regulatorischen Umfeld (Quick-Check Compliance), Ausarbeitung der Handlungsbedarfe, Einbeziehung von Best-Practices.
Lösungsvorschlag mit Digital Health Regulatory Suite
Erstellung kundenorientierter Lösungsvorschlag: Einführung von einzelnen Solutions der Digital Health Regulatory Suite anhand der Kundenbedürfnisse, Integration in die bestehende Landschaft, Betrachtung von Kosten/Nutzen. Erstellung einer Umsetzungstimeline anhand der regulatorischen Erfordernisse.
Implementierung Digital Health Regulatory Suite
Auslieferung vorkonfigurierter Solutions z.B. für das Qualitätsmanagement. Weitere Unterstützungen der Implementierung, z.B. bei der Aufnahme und Dokumentation von Prozessen sowie der relevanten Kontrollen. Weitere Integration in die Systemlandschaft, z.B. durch Anschluss einer bestehenden CMDB Lösung.
Weitere Zeritifzierungsunterstützung
Wir unterstützen unsere Kunden nicht nur durch weiteres Customizing und Konfiguration der Softwarelösungen, sondern auch operativ im Bereich der für die Zertifizierung notwendigen Tätigkeiten. Der Kundenvorteil besteht in unserem Expertenwissen im Bereich leaner Prozessgestaltung, End-to-End Prozessketten und Wertschöpfungsflüsse, Supplier-Management oder Informationssicherheit in der kritischen Infrastruktur.